the Shapes of the Biomolecules... determines how molecules interact with other molecules. It is the weak molecular forces that shape molecules and build Molecular Complexity & Biological Activity & Life. |
 |
|
Biological work entails mechanical functions, biosynthesis, transport, electrophysiology, homeostasis, etc... all of which depend upon the SHAPE of MOLECULES. |
|
|
Structural Chemistry: 3D-molecular shape is dependent upon the orientation of covalent bonds in space. Molecular Configuration: results in specific bond angles & molecular geometry |
|
|
methane CH4 109.5o - a tetrahedron with free rotation formaldehyde H2C=O 120o - same plane with no free rotation |
|
| One key to understanding molecular shapes is seen in the ASYMMETRIC [chiral] CARBON ATOM... | |
|
a carbon atom bound to 4
dissimilar atoms in a nonplanar configuration often forms molecules based upon a tetrathedron* shape [ water + other examples*]... |
|
| |
|
Asymetic
carbons result in molecules that differ in
3D-orientations in space i.e.,
STEREOISOMERS...
or optical isomers*,
molecules,
which have identical composition, but are not
equivalent, as they
have different molecular orientations in
space or are MIRROR IMAGES...
such
chiral molecules are not
superimposable on its mirror image...
as with human hands. They are also
called ENANTIOMERS*.
Stereoisomers may have
mostly identical
chemical properties, but do show an
unusual optical property: the isomers rotate plane of
polarized light at different
angles.
LEVOROTARY (L
or S)
- rotates light left (- negative optical rotation)
DEXTROROTATORY (D or
R) - rotates light
right (+ positive optical rotation)
Stereoisomers can
often have different BIOLOGICAL ACTIVITY: some
examples...
a.
dihydroxyphenylalanine [L-DOPA] fig* --> Encephalitis lethargia & "awakenings"
b. sedative thalidomide* = severe birth
defects due to L
(or S isomer); D or R helps nausea
Gruenenthal
Pharmaceutical Group apology - statue.
 ???
Which do you think would be more thermodynamically
stable and why?
???
Which do you think would be more thermodynamically
stable and why?
a homochrial
polymer (made
of same enantiomer isomer - say all D)
vs. a heterochiral polymer (made of a mix of
enantiomer isomers - D & L) ?
![]()
Biological activity... is the catalytic ability
of molecules to
do work.
There
are 2 structural properties of
biomolecules,
which contribute to a
molecule's unique Fitness for
Biological
Activity & the Living
State.
![]() *
*
1st.
CONFIGURATION: the permanent geometry that
results from the spatial arrangement
of a molecules bond's, i.e., the spatial
arrangement of atoms via bonds
in the
molecule that may not be
inter-converted without
those breaking bonds.
Some example of covalent bond molecular
configurations...
ISOMERS... based upon covalent bond
orientations [glucose
vs. galactose]*
and each has different chemical &
biological properties.
ISOMERS...
built
upon planar covalent double bonds*
-C=C-
fix atoms above & below plane of molecule (planar) &
restricts free rotation, thereby fixing a 3D shape in
space.
Cis vs. Trans*
maleic (cis)
vs. fumaric (trans)
![]() 11-cis-retinal
vs. 11- trans-retinal*
11-cis-retinal
vs. 11- trans-retinal*
Biological
Activity
& the
Shape of Biomolecules
continued...
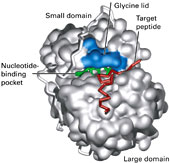
| 2nd. CONFORMATION [or shape] - is the surface outline or contour of molecules | |
| - is 3D orientation
of all chemical groups in
molecules free to assume different positions in space without breaking any bonds - do primarily to... FREE ROTATION of atoms about a single chemical bond & WEAK NON-COVALENT FORCES hold atoms in spatial arrays |
 |
|
-
consequence of conformations... different conformational shapes (forms) of molecules can exist, only one of which may be biologically active (the other conforms aren't) ENZYMES can distinguish between biologically active forms (CONFORMS*) based upon the "SHAPE" of that molecule. |
|

|
|
| Molecular Conformations are due to the Weak Molecular Forces* of a cell's environs... |
|
IONIC
bonds* attraction between cation (+) & anion (-); no fixed geometry
for electrostatic field-uniform in all directions; readily soluble with polar water [Na+,K+,Ca+2,Mg+2,Cl-] |
|
DIPOLES*
attractions via asymmetrical, internal distribution of
charges in
a neutral, molecule, one which has no net charge (opposite poles +/- attract weakly) |
|
DISPERSION* (van der Waal’s) Forces-
electrostatic
interaction between orbitals of 2
atoms... generates transient dipoles that attract/repel; results in cohesion between non- polar molecules that don't form H-bonds mcb fig 2.10 important in 3-D shapes |
|
HYDROPHOBIC Interactions* - repulsion of electrostatic
dipoles of water by non-polars- "fatty-hydrocarbon" groups can self assembly - "like dissolves like" |
|
HYDROPHILIC
Interactions* -
substances that dissolve readily in water
(ions & polar molecules) water, as a dipole, surrounds & solubilizes a solute molecule |
|
HYDROGEN
bonds* electrostatic attraction
between H of one atom &
pair of non-bonded e-’s on an acceptor group; linear directionality. OH & N-H with O- & N- |
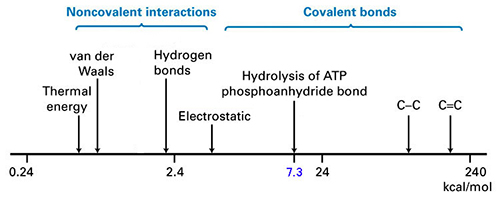
| Non-covalent Electrostatic forces in action*... (all are in the 1-10 kcal/mol range) |
| |
Covalent vs. Weak
Molecular Forces of Life ...
| TYPE of BOND | ENERGY (kC/mol) | TYPE of INTERACTIONS |
ENERGY (kC/mol) |
| SINGLE COVALENT BONDS | NON-COVALENT BONDS (in water) Tbl 2-1 | ||
| O - H | 110 | IONIC BONDS | 2.5 -
3.5 |
| H - H | 104 | HYDROGEN BONDS | 0.5 - 1.5 |
| C - H | 99 | HYDROPHOBIC | 1.0 -
2.0 |
| C - O | 84 | VAN DER WAALS | 0.1 - 0.5 |
| C - C | 83 |  |
|
| S - H | 81 | ||
| C - N | 70 | ||
| C - S | 62 | ||
| DOUBLE BONDS | |||
| C = O | 170 | ||
| C = N | 147 | ||
| C = C | 146 | ||