|
The
Design of Metabolism...
|
Metabolism - Gk metabole
- change
Energy
Transformation of Glycolysis
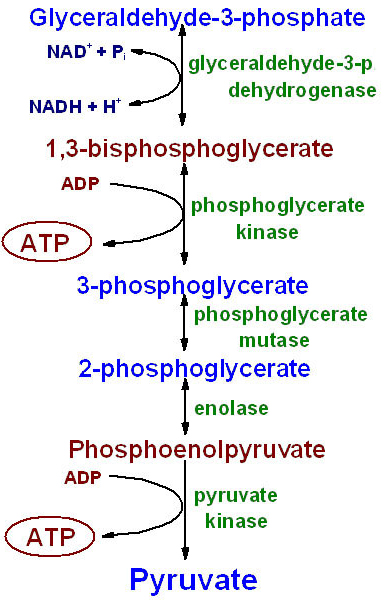
|
|
& are able to Transform Energy
|
|
|
ENERGY in cells is housed in a
molecule's
CHEMICAL
BONDS...
- Cells
possess "Chemical
Potential Energy"
Cellular Energy
also occurs in such forms as:
- chemical
concentrations gradients
across membranes
can diffuse from [higher] to [lower]
- electrical gradients (potential differences)
across membranes
a separation of charge
as much as 200,000 volts per cm2
Cellular Transformations
of Energy make
up metabolism
& the cell pathways
and enzymes that run cell pathways.
|
|
 ecb/5e - chapter 3 ecb/5e - chapter 3 |
ENERGY IN ----> CELL METABOLISM ----> ENERGY OUT
We need to be able to Measure Energy
Transformation, esp. Energy's
ability to do work...
Willard
Gibbs (1839-1903) applied the principles
of
Thermodynamics to chemical
systems
& determined
the energy content & changes within a chemical
reaction. He proposed the ...
FREE ENERGY EQUATIONS
ΔG
=
ΔH
- T ΔS
 * *
free energy*
enthalpy
entropy*
ΔG
= ΔG0’ + R T ln [products] /
[reactants]
ΔG is measure amount
energy in system able to do work (to stay
away from equilibrium)...
Disorder increases (thus entropy
increases) when
useful energy,
that which
could be used to do work, is dissipated as heat...
Biological systems are ISOTHERMAL, e.g., held at constant temp/pressure
(4o to ≈ 65o ) and thus in
biological systems ΔH
≈ 0
Cellular
systems
tend to break down (become disordered* ) & their potential/kinetic
energy
is converted
into heat... Cells
combat this slide toward disorder by continually
consuming new energy
(from sun) to repair/replace structures
broken down.

|
Predicting*
which way will this reaction go?
[step 5 of glycolysis]
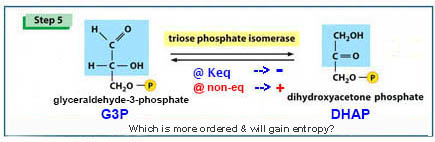
|
|
GIBBS FREE ENERGY EQUATIONS*...
ΔG =
ΔG0’ +
R T ln [p]/[r]
and ΔG0’
= -RT ln Keq
[ -(2.0) (296)
(2.303) lg10
Keq ] |
|
Which way
this reaction will go will dependent
upon standard or
existing
conditions ? |
|
ΔG0’
@ [equilibrium*]
the
Keq of
DHAP/G3P is shown to be 22.4
ΔG0’
= - [1364] lg10 22.4 = - [1364]
(1.35) = -1,842
cal/mole (R → L) |
|
But... ΔG
= ΔG0’ + RT ln [P] / [R] when Keq
= 0.01 [DHAP = 0.1M
& G3P
= 0.001M]
ΔG = -1842 c/m
+ (-1364) (lg10 0.01)
=
(-1842) +
(-1364)(-2) = + 886 cal/mole |
|
Thus under standard condition the reaction is favored
from G3P toward
DHAP (-ΔG),
but under a specific cellular
condition,
where the ratio of reactant &
products is changed,
the
reaction may not be favored, and can go in
the other direction from DHAP to G3P...
This is what happens in glycolysis*, the pathway shifts R/P ratios and pulls rx to G3P.
So
How does Metabolism create more order when
many reactions are endergonic?

|
-
-
-
-
-
-
-
-
-
≠≠
How does Metabolism create more
order using cellular chemical reactions?
Many biological
systems can increase order... i.e., a decrease in entropy
( ΔS < 0) via...
|
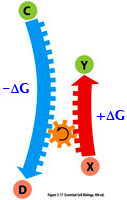
COUPLED
REACTIONS - often
involve... the linking of the hydrolysis of
ATP
(a favored reaction) to a
thermodynamically non-favored reaction,
thereby creating some biological
order (greater
molecular structure).
ΔG
for the reaction X
--> Y is +, but is less
than the ΔG of C
--> D
for example, via hydrolysis*
of
ATP*
and/or phosphate transfer*
then
the reaction may be driven to
completion by coupling to ATP hydrolysis
synthesis of
glutamine* by
glutamine
synthetase
synthesis of
sucrose*
by
sucrose
6-phosphate synthase |
 |
most
cells use ATP
hydrolysis energy and couple it
to processes as:
conformational
changes in enzyme, like kinases*, which phosphorylate
proteins converting then from inactive to
active (& vice versa);
energy gained in the stressed conformation
is released,
when the protein relaxes.
the ability to couple reactions is
one of the unique properties of living
organisms...
and
How does life stay away
from Equilibrium* ???

|
|
|
"Secret of Life is not
some "vital force", but the unique energy
transformations
of the
Second Law of Thermodynamic at the molecular
level. "
Energy is the currency of biology. By
harvesting electrons
from covalent bonds in a range of molecules,
living organisms produce adenosine
triphosphate (ATP),
which powers biological reactions.
In the case of
mammals and most
eukaryotes, sugars
and other organic
molecules are common
electron sources, the oxidation of which
drives ATP production. Bacteria &
Archaea can use a range
of other chemicals, from sulfide
to iron
to ammonium.
Cells take up
these electron-rich molecules and capture
their electrons, which flow down an electron
transport chain in the
mitochondrial or cell membranes. As
electrons move along the membrane toward
a final electron acceptor, protons are pumped across
a membrane setting up a chemical gradient.
Finally, protons stream back
across (via ATP synthase)
releasing the chemical pressure and
generating
ATP.
With each energy-requiring reaction, from
flagella construction to cell division and
growth, cells
draw upon their ATP bank.
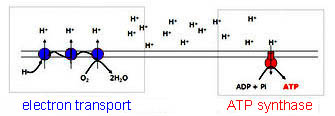
So exactly how
do cells capture electrons to make ATP?
 next lecture-Making ATP next lecture-Making ATP
|
SKIP
this PAGE SCIENCE of ENERGY TRANSFORMATIONS
is THERMODYNAMICS |
1st Law of Thermodynamics... Energy can neither be created
nor destroyed,
but is
convertible. nuclear
blast -
mass of U235
--> heat/light
photosynthesis
--> sunlight into glucose bonds
muscle -->
hydrolysis of ATP into contractions |
all
forms of energy are inter-convertible thus all
are expressed in same
units of measure
Joule, but biologists use more
common calorie [heat ↑ 1gm 1oC]
1 Kcal = 1,000 cal =
4,186.8 Joule
[1 cal =
4.1868 J]
2nd Law of Thermodynamics…
ENTROPY
is commonly referred to as a
measure of degree of
order of the Universe,
and
thus its randomness;
ENTROPY
(Disorder) CAN ONLY
INCREASE
The Rules
of the Universe are simple:
Cities crumble, Stars go
Supernova, and we are all
equlibrium...izing (dying)
Entropy* is
maximum disorder..... "heat" 
 Events in the Universe have
a direction ---> to
MAXIMIZE ENTROPY Events in the Universe have
a direction ---> to
MAXIMIZE ENTROPY
|
SKIP this PAGE
What Gibbs showed was that...
"cell chemical systems proceed so
that Free Energy is minimized"
- the
Direction of Cellular Reactions......
should proceed
TOWARD EQUILIBRIUM
and toward Maximum ENTROPY...
and measurement of
the ΔG can
help PREDICT* the likely direction of
reactions...
Any natural process occurs
spontaneously,
if and only if,
the associated change in G for the system is NEGATIVE (-ΔG < 0).
when
-ΔG
is negative a reaction is SPONTANEOUS,
R --> P,
& there is a increase in
entropy
Likewise, a system reaches
equilibrium when the
associated change in G
for the system is zero (ΔG =
0),
and
no spontaneous process can occur, if the change in G is POSITIVE (+ΔG > 0)
unless energy is put into that process.
-

-(2.0) (296) (2.303) lg10 Keq
"Life is like
a Casino"
|