Life is thermodynamically far from equilibrium...
...and chemical reactions proceed from a
disequilibrium state toward equilibrium...
Life can exist as such because the downhill (exergonic
reactions - release free energy) of molecular degradation,
which move toward equilibrium are slower than the uphill
(endergonic - energy requiring) reactions...
exergonic reactions are slower than
endergonic reactions.
endergonic reactions.
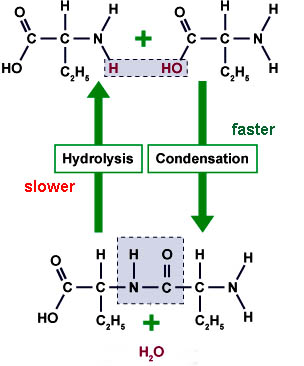
activation energy needed, while
condensation reactions are faster,
due to lower activation energies.
thus complex molecules may exist in a metastable
state for extended periods of time (Life).
back