The main difference between L, D configuration and S, R configuration is that the first one is relative configuration while the second one is absolute configuration.
When you are distinguishing L-alanine from D-alanine, you only know that the
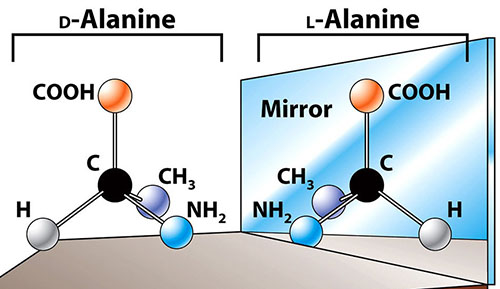
group on the chiral carbon of L-alanine is on the left hand side, while in D it is on the right hand side relative in a Fischer projection. There you can't be specific about other functional groups (like or ) position.
But when you distinguish S
form of Alanine from the R
form, it is done by proper arrangement of the other two
functional groups and a hydrogen atom. If any one of those is
switched to some other position, the absolute
configuration may change from S to R
or from R to S. So, the
configuration is more rigid in case of R-S
configuration.
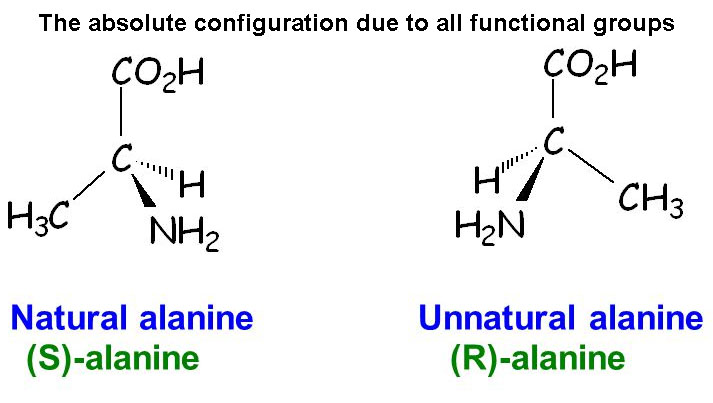
As the L-D configuration doesn't specify other group's position,
you can't always say S-alanine instead of L-Alanine
unless you know total spatial arrangement of all functional groups
of alanine.