The chemical conditions of primitive Earth may have lead to
the Origin of Life?
To know how simple molecules in the
early oceans & atmosphere become cells?
What are the Chemical Properties
of the Biomolecules of Life?
We hypothesized that "Cells might be be
called... Chemical Machines"...
for cells are
made of MOLECULES &
molecules are made of MATTER
|
matter
--->
molecules
---> cells

|
MATTER : physics
defines it as anything that occupies space &
has
Mass
the mass of an object is the number of atoms the object
has in it.
|
Mass is often equated to weight, but weight & mass are not equal:
weight
is due to pull of gravity; mass is amount of matter in an object
(150 lb person on moon
weighs 25 lb, and
on a neutron star = 21
trillion lb) |
Matter is composed of
the ELEMENTS
of the Periodic Table. |
| |
 next panel next panel |
-
-
I. ELEMENT - is a pure substance
that contains only one type of ATOM...
-
Atoms
of each element has an
identical number of PROTONs
in
their nucleus.
-
Atoms CAN NOT
be reduced to simpler substances
by normal chemical means.
There are 92
naturally occurring
elements and 112 IUPAC
recognized
elements all arranged in a Periodic Table*
Periodic
Table of Elephants
|
an ATOM -
is a unit of matter
that has a
particular structure... |
-
- it's the smallest unit of
an element, having all the
-
characteristics of that
element & consisting of a dense,
-
central,
positively charged nucleus surrounded by
-
a system of negative
electrons. The entire
structure has
-
an
approximate diameter of 10-8
cm and characteristically
-
remains undivided in
chemical reactions except for
-
limited removal,
transfer, or exchange of certain e's.
|

click on image |
 next
panel next
panel |
-
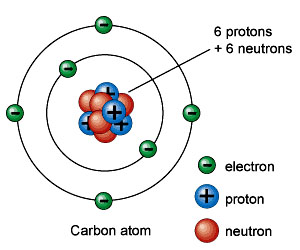
2. Structure of an Atom: at
the center of an ATOM
is its NUCLEUS,
which has 2
fundamental sub-atomic
particles:
-
PROTON
[mass - in
grams =
1.673 x 10-27grams]
- + charged particle
- #
protons present defines the ATOMIC NUMBER
6C
helps define the chemical
properties of that element
|
 click on image
click on image
|
- NEUTRON
has equal mass [1.7
x 10-27grams]
as a proton,
-
but has a NEUTRAL
electrical charge
|
an atom's
NUCLEUS in the
Niels
Bohr Model  animation* animation* |
-
 next panel next panel
-
the 3rd fundamental particle is the ELECTRON
hypothesized in
1892 by Dutch physicist Hendrik
Antoon Lorentz.
In 1897, J. J.
Thomson proposed the neutrality of
atoms when he proposed a model
of the atom with negative electrons
scattered throughout a sphere of positive
charge.
Fundamental Properties of
Electrons:
1. has a much
smaller mass [1/1,836th of proton]
2. is
NEGATIVELY charged
- in
non-ionized atom # of electrons = # of protons
3. obey the Law of Charge Conservation
and do not spontaneously decay (live forever)
4. has intrinsic Spin (and may
provide a sense of compass
direction*)
5. surrounds
an atom's nucleus in an Orbital Cloud*
cloud: area where electron
is likely to be found
space: if e- were size of an apple, then
shell's orbit = a 1 mile diameter
Helium atom - animation*
Orbital
Stability... is
achieved when all the
Subshells are filled with
electrons resulting
in
in the stable electron arrangements of 2, 10
(2+8), 18 (2,+8+8), etc...
Na with atomic # of 11 = 2, 8, 1 electrons in Outer Subshells* & subshells of Al
& Cr
Valence Subshell... the OUTER ORBITAL
containing Valence
Electrons*...
valency is
the number of electrons required to complete an
atom's
outermost (valance) subshell by forming chemcial
bonds with another element.
An element chemical reactivity* depends
upon the degree to which outer shells is filled.
 next panel next panel
CHEMICAL
REACTIVITY comes about mainly
because some electrons are easily
attracted AWAY FROM or
attracted TO the outer
orbitals of other elements;
electrons are directly involved in chemical
reactions to form chemical bonds...
-
|
|
- ION... an atom
or a group of atoms that has acquired a net electric charge
-
by gaining or
losing one or more electrons.
|
|
Sodium (Na - from
Latin natrium)
has an atomic # of 11
& mass # of 22
(11 protons & 11
electrons).
easier to lose 1e (2,8,1) (= Na+
ion)
A stable isotope is Na 23
with 11 protons
and 11 electrons, but
12 neutrons.
|
 |
|
 cartoon
next
panel cartoon
next
panel
|
|
|
-
ISOTOPE...
atoms with same
# of protons but MORE neutrons
(it has a greater mass)
- atomic #
= number of protons in an atom
- atomic
mass = number of protons + neutrons (often called atomic weight)
-
comparison of how heavy one atom is to another is
expressed
-
as AMU, atomic mass units, also called
DALTONs (Da).
-
all atoms were originally compared to hydrogen = 1.0079amu
but now, 1 amu = 1/12th of mass of carbon
=
1.660 x 10-27gm
- among
all naturally occurring Carbon
atoms...
99%
<
carbon-12 atomic
number 6C12
atomic mass 6p + 6n
-
< 1%
< carbon-13 atomic
number 6C13
atomic mass 6p + 7n
-
< 1%
<
carbon-14 atomic
number 6C14
atomic mass 6p + 8n
-
- Unstable isotopes undergo
spontaneous radioactive decay* of the atomic
nucleus giving
off subatomic
particles, energy & leaving 1 more proton...
with a constant half-life decay*
Sr 90 = 28.6y
Cs
137 = 30.2y
H3 =
12.3y
- C 14 = 5,730y [C14dating*]
U
238
= 4.5 billion y [ U238 decay ]
Uses of ISOTOPES:
an example of tracing a biomolecule
during a metabolic process*
 next
panel next
panel
-
II. Molecules... Life is definable by the forces
(chemical bonds) that make Molecules:
Chemistry
boils down to changes in the electronic
structure of atoms and then molecules, i.e.,
the
processes by which changes occur in electronic
structure of atoms that form
compounds
with
new emergent properties i.e., groups of ATOMS [2 or more]
held together by
"energy" in form of a "chemical
bond"
Electronegativity*
- atoms ability to attract
electrons to itself when bonded to
another atom.
Types of Chemical Bonds:
Make a List*
- Ionic bond... an
attraction between atoms of opposite electrical charge +/-
- Na
with 11 e's it has 1 outer unpaired
electron
11
Na (2,8,1)
- needs a
full orbital of e- to be
stable..., could gain 7 more e-
- thermodynamically
easier to lose its 1 e- to achieve
electronic stability
- leaves +
charge (1
extra proton) in the
atom... thus --> CATION (+)
.
- Cl
has 7 outer e-'s 17 Cl (2,8,7)
- needs only
to gain one e-
to become stable, so favored energetically,
- giving an
extra -
charge (extra
electron) in the atom...
- thus
---> ANION
(-)
 animation on ions*
Iionic
bond image*
NaCl ionization* animation on ions*
Iionic
bond image*
NaCl ionization*
Na+
& Cl-
is a good example of
molecules with emergent properties*
-
-
-
-
-
a Covalent Bond*...
thermodynamic stability is achieved via atomic
orbitals merge into
one "molecular orbital"
that extends over more than one atom; i.e.,
by sharing electrons between 2
atoms...
-
American chemist Linus Pauling
(1939) first proposed that a covalent
bond forms when the the electronic area of
different atoms overlap in space.
Robert Mulliken & Frederick Hunds (1966)
hypothesized "molecular orbitals".
- two atoms share atomic
nuclei via their outer
e- orbitals
- results in orbital stability for
each, thus energetically
favored
 next panel next panel
Molecular
Architecture of Cells
the Structure of Cells in
Chemical Terms
the Key Concepts*
Some Salient Features of Chemical
Nature of Cells:
70% of mass of a cell is water (H2O),
i.e., 30% is non-water
extremes... dry plant seeds < 1% water
ctenophores & coelenterates
> 95% water
"chemistry of life
is often described as the chemistry of water"...
- or
solution chemistry.

Role of water & its location
in cells...
1.
the cytosol is described as SOLUBLE
PHASE of the cell - but, where is the water?
we assume cell is full of water,
but... Look at some EMs* & the cytoskeleton*
classical paradigm --> a living cell is an
aqueous membrane sac
with stuff
floating in a water based compartment.
"a molasses filled
balloon with floating ping-pong balls"
BULK* vs. VICINAL
interfacial water, i.e., solutions
vs. water at an interface*
interfacial
water has different chemical &
physical properties,
but, we will continue to assume that
cells function on the bulk properties
of water.
2.
thus, water is solvent for chemical reactions in a test tube & supposedly
in cells...
water is the oxide of hydrogen
H-O-H
3.
thus, water is SUBSTRATE for and PRODUCT of many enzymatic reactions...
6
CO2 + 6 H20
<-----> C6H12O6 + 6 O2
4.
and water plays a
structural role, especially with large
macromolecules...
... it
hydrates DNA, RNA, proteins & enzymes
 next panel
... it
adds form & dimension to many molecules
HOW? next panel
... it
adds form & dimension to many molecules
HOW?
-
Emergent
Properties of water favoring Life:
Water
exits in
3
forms*: gas liquid solid due
to its molecular
arrangements
- - it is a liquid at ambient [room] temperature
(not
alot of molecules are)
- has high surface tension... a measure of
cohesiveness* of water for itself.
how does water get to the top of tall
trees?
--> transpiration
-
only metal Hg has a greater surface
tension --> water bugs
-
- has high specific heat...
is the amount of energy [heat being
absorbed]
-
needed to raise 1 gm
water 10C
1 cal = 4.186 joule/gm
- water's
specific heat is as high as many
oils/alcohols, higher than some metals
- provides
good thermal
insulation...
pics ( Gulf
stream - temps
)
-
- has
high heat of vaporization
amount of heat energy to convert LIQUID to GAS.
- for water it's 540 cal/gm - greater than ether or
ammonia
-
evaporation
of sweat... releases 540 cal of heat per
gram [ml*] of
vapor/sweat
-
-
heat of fusion is 79.7
cal/gm...
freezing water converts molecular motion of
liquid water
into solid & releases heat energy during the
phase transition, and vice versus
solid ---> liquid = +
80 cal/mol
liquid --->
solid = - 80 cal/mol
(graph)
ice melts in scotch rocks when heat is absorbed
from the surroundings.
- on freezing...
as a solid water is LESS
DENSE* increases its
volume.
liquid
water is 10% more dense that ice
-
Water is Weirdanim
- why is it such a strange molecule? *
-

-
Structure
of Water
...defines the
physical properties of water and of many biological
molecules.
Oxygen =
8O
[2,6]
i.e., needs to gain 2 e- to be more
stable...
Hydrogen = 1H &
1H i.e.,
each need to gain 1 e- to be more stable...
so, let's put them
together:
atoms = one
Oxygen & two Hydrogens
each share 1 e-
forms 2 covalent bonds [ H-O-H ]
bonds that form a
tetrahedral shape*
-
Tetrahederal shape of water
bonds makes water a Polar Molecule
properties of
water - anim*
& non-polar
vs. polar covalent bonds animation
i.e., water shows an unequal distribution of internal charge -->
polar bonds*
Water is Molecular Dipole (magnet-like) with two opposite
charged ends in the same molecule.
slightly + on one side &
-
on other side ; binds to NaCl* &
protein*
the
dipole of water makes water a great Solvent...
hydrogen bonds
provide structure to biomolecules*
 weird
factoid: 1 cubic mile of ocean water
holds 400 lb of gold (but not really
extractable) weird
factoid: 1 cubic mile of ocean water
holds 400 lb of gold (but not really
extractable)
Often molecular
structure in cells depends upon...
Hydrogen Bonds... which is responsible for the
structure of water.
H-bonds are weak electrostatic
attractions between water molecules
and/or any molecule containing a dipole
(such
as COOH & NH3)...
electropositive end of
one H2O is electrostatically attracted
to electronegative
end of another H2O or another
dipole.
not really a "bond"*
at all,
but rather a weak
electrostatic attraction
Thus: All the properties of water stem
from its H-bonds --> properties*
There is no life
without water: and water's
properties may be
one reason that the molecules of life formed in
the first place.
 key concepts*
Ray
guns key concepts*
Ray
guns
back
next lec
copyright
c2024
Charles Mallery,
Biology 150, Department of Biology, U.
of Miami, Coral Gables, FL 33124
|









