|
CHEMICAL ARCHITECTURE of CELLS...
if 70% of a cell is
water what's other 30% ?
|
|
it's the Elements
and Molecules within CELLS...
96% of mass of
cells (i.e., the other 30%) is ONLY 4
ELEMENTS tbl 2.1*
- C H O N + P S
[ old
mnemonic---> "C.
HOPKIN'S
CaFe Mg" ]
- Why only
these lelements ? their valances favor
covalent bond reactivity*
- they readily form molecules due to
their electronegativity.
- these elements make up
the inorganic and organic molecules of cells:
Cellular elements, plus many METAL IONS,
responsible for many reactions:
1. make up parts of
organisms as bone, exoskeletoms, and
vitamins.
some examples:
Mg in chlorophyll,
Fe in heme, & nickel in enzymes.
2. are dissolved in aqueous
media of the cell and serve
as catalysts
for many chemical reactions.
|
 |
Metabolome is
the complete set of small molecule metabolites
occurring in an organism (akin to the term 'genome').
.
- metabolome of plants = 50,000
components
- estimates in Human
= 40,000+, & some 2,080 enzymes, & 115
pathways
the human metabolome
database
- the vast majority of
all metabolome components are:
ORGANIC Molecules...
molecules
composed primarily of elements of C & H,
are the building blocks*
of polymeric biological molecules.
the
macrolmolecules that make up cells & Life
MOLECULES*
BIOLOGICAL ROLE
carbohydrate
(CH2O)
structure & energy molecules
proteins
(CHONS)
structural, enzymatic
(catalytic)
nucleic
acids
(CHONP)
informational, genetic
role
fats (lipids)
(CHO)
structure & energy molecules
phospholipids
(CHO-P)
membrane structure
steriods/sterols
(CHO)
membrane structure
- hormones
 Why is it that
organic life is based upon Carbon and not
Silicon? Why is it that
organic life is based upon Carbon and not
Silicon?  es 6
es 6
Some Basic Bio-Organic
Chemistry...
including
the ways to represent molecules graphicallyin
texts and figures...
Molecular (or Empirical) Formula
vs.
Structural Formula
...stick and space filling
models
fig 4.3*
&
3D molecular models
- Hydrocarbons -
molecules made of Carbon
& Hydrogen
& have molecular
skeletons*:
- ...some
examples : chains
vs. rings
fig
4.5*
-
ISOMERS - structural or
fig
4.7a*
-
...have the
same empirical formula, but different structure
- geometric - cis-trans isomerism
fig
4.7b*
... differ in arrangement of groups attached to
planar C=C
- optical
fig
4.7c*
... bend plane of
light is different directions (mirror images)
summary of all isomers = fig 4.7
-
A. CARBOHYDRATES... are polymers
of simple sugar molecules, which
have a consistent ratio of [CH2O]n glucose - C6H12O6
or galactose - C6H12O6
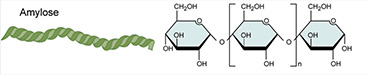
-
1. MONOSACCHARIDES... or the simple sugar monomers,
-
are the monomers
that make polymers as starch, glycogen, &
cellulose
-
(single molecules)
(polymers)
-
- Chemical
CLASSES of Monosaccharide monomers:
- built upon the chemical groups of an
aldehyde or a ketone
- aldoses [built on an aldehyde] vs. ketoses [built on
a ketone]
- -
names based on # C's - trioses,
pentoses, hexoses
fig 5.3*
-
Structures of Monosaccharide Glucose:
Glucose - straight chain vs.
ring structures*, [glu images*]
abbreviated ring*, optical
isomers*,
other isomers*,
α & β isomer forms*
-

-
-
-
DISACCHARIDES...
are composed of only 2
monosaccharides...
formed
by condensation rx
----> polymers*A
videos:
disaccharides*A
---->
GLYCOSIDIC bond*
-C-O-C-
| |
|
|
|
| |
dissacharide |
bond |
polymer |
| |
maltose*
|
α-1,4 glu-glu
|
---> amylose |
|
sucrose*
|
α-1,2
glu-fruc |
---> sugar |
|
cellibiose* [3D]
|
ß-1,4 glu-glu |
---> cellulose |
[animation -
descriptions of carbs]
View@home

-
POLYSACCHARIDES...
the complex sugar macromolecules
are
-
... polymeric chains of many
monosaccharides linked together.
-
... forms long repeats in helical shapes...
(like a staircase)
-
- STARCH... a
polymer of glucose monomers in the
α-1,4glu configuration:
-
also know as... AMYLOSE
is an unbranched polymer
-
AMYLOPECTIN
is a branched polymer
GLYCOGEN*...
multi-branched polymer [helps solublize it in
human & animals]
-
CELLULOSE*... unbranched glucose polymer of ß-glu (for ex: in
leaves*)
animals lack cellulase but symbiotic bacteria can
digest cellulose
-
CHITIN*...
exoskeleton of
insects & crusteceans,
adds +NH2
group
Review of
Carbohydrate StructuresView@home
& tutorial on carbohydratesView@home
-
the take home message here is ...
►
"MOLECULAR
STRUCTURE is
CRITICALLY IMPORTANT to FUNCTION, &
-
STRUCTURE
relates to the 'ORIENTATION' of covalent
bonds in 3D space"

Importance of Molecular Shape to Biological
Activity
2 unique
PROPERTIES of BIOMOLECULES gives them special
FITNESS
for Living State...
1. Configuration
- is
the PERMANENT GEOMETRY of a molecule that
results from the
spatial arrangements of its
COVALENT BONDS in
space:
Configurations can't be inter-converted without
breaking chemical bonds &
often
involves an asymmetric
Carbon atom... (carbon
w 4 diff. groups attached*)
ex: STEREOISOMERS,
also called enantiomers have same molecular
formula
but differ
in 3D orientation of atoms in space and are mirror images, which
are
not superimposable
upon each other thus they exhibit
property called CHIRALITY.
a unique property of amino
acid stereoisomers with identical
chemical structures,
is that they rotate plane
of polarized light at different
angles...
levorotatory [
L ] or [S]
= left handed
- counter clockwise
dextrorotatory [ D] or [R]
= right handed -
clockwise
molecular
examples: amino
acids & glucose & limonene*
medical ex: Parkinson's
disease and an L-DOPA
treatment -[Animation*
& Awakenings]
(L-DOPA* -DihydrOxyPhenylAlanine) is converted to
neurotransmiiters
as dopamine, which reduces the symptoms of
Parkinson's
-
-
-
-
-
-
-
-
- Configurations - permanent geometry due to
spatial arrangement of bonds:
examples: are Structural Isomers, as pentane and isopentane.
but often the
best examples
are seen with cis-trams isomerization, isomers that
are based upon the presence of "covalent
bonds", especially
carbon-carbon
double bonds C = C
-
Double bonds fix
atoms in one plane = above
& below
plane of molecule
removes ability of groups attached to C-C bond
to freely* rotate
the C=C bond makes the
molecule Planar C=C* 3D* no
free rotation
-
-
examples:
Configuration (geometric)
Isomers... &
cis and trans configurations
cis
vs. trans
figure*
a biological example of Cis
& Trans isomerizations:
11-cis-retinal vs.
11-trans-retinal retinal*
other example of
configuration differences
= functional differences:
-
estradiol
vs. testosterone
figure*
-

-
-
-
-
-
-
-
-
-
-
2. Conformation - [3D-shape]... the
surface outline or contours...
3-D
orientation of a molecule, which
results without breaking any covalent
bonds |
due to
free rotation [360o]
of atoms about
a single chemical bond
& the weak
electrostatic forces holding
molecules together... figure* |
►
STRUCTURE, SHAPE
and
FORM
and
BIOLOGICAL
ACTIVITY... |
Molecular
shape is CRUCIAL
in biology, because it determines how
biological
molecules recognize
& respond to
one another with Specificity.
Molecules with
complimentary shapes can form weak bonds with
each other:
ex: endorphins - include a group of
nervous system & brain hormones
(dopamine, serotinin, oxytocin) that bind to
analgesic receptors promoting
positive feelings & reduce the
perception of pain.
thought question*
: how do opiates like morphine & heroin
work on the brain?
|
-


B. FATS and LIPIDS...
(another macromolecue)
a TRIGLYCERIDE
or Fat or Triacylglycerol...
animation of fat structure*
a triacylglycerol is formed
by condensation reaction of
1 GLYCEROL
and 3 FATTY
ACIDs
figure*
PROPERTIES... structural
figure*
glycerol end of fat is............HYDROPHILIC (POLAR - attracts water)
hydrocarbon end of fat is....HYDROPHOBIC (NON-POLAR -
repels water)
Saturated*
(solids) vs. Unsaturated
(oils)* cis-trans
fats* - properties*
fats vs. carbs* as energy
sources
a
PHOSPHOLIPID... (basis of membranes)
1 glycerol, 2 fatty acids, PO4,
& an organic
molecule P-lipid*
Properties
MICELLES & BILAYERS
amphipathic*
CHOLESTEROLS...
classified as lipids
because they're
insoluble in water
(13 Nobels) cholesterol
& anabolic steroids figure* &
in membranes*
 [animation
describing all types of lipids]
View@home
[animation
describing all types of lipids]
View@home
C.
NUCLEOTIDES...
-
the organic monomers
[ATGC &
U] of the nucleic acids
are composed of 3 parts...
a nitrogenous base*, a
ribose (5C) sugar*, a phosphate* (-PO4)
they are part of the cell's
energy nucleotide - adenosine triphosphate -
ATP*
and form
a part of polymer*
of single nucleotides [ATCG
(U)] made by
linking the
nucleotides together via phosphodiester
backbone*
RNA
- ribose nucleic
acid
- (single stranded)
DNA
- deoxy-ribose nucleic acid
-
polynucleotide building
blocks of DNA & RNA*
DNA double helix
of 2 polynucleotide chains* & animation*View@home
 bonds we have
learned to date*
bonds we have
learned to date*
-
FUNCTIONAL GROUPS... (key to understanding
the chemistry of biological reactions):
... groups of atoms
acting as a unit,
-
... give organic molecules their physical properties,
and their chemical
reactivity, & solubility in aqueous
solutions.
most possess electronegative atoms (N, P, O,
S... EASILY
ATTRACT PROTONS)
key bonds are : ester (C-O-C=O) & amide (O=C-N-)
are ionizable at
physiological pH.
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Consequences of Substitution of a H
with a
Functional Group
...Linking STRUCTURE,
EMERGENT PROPERTIES, and BIOLOGICAL
ACTIVITY ?
-
Ethane
CH3-CH3
toxic, flammable gas
- Ethanol
CH3-CH2-OH
ethyl alcohol, a potable drink
- Propionic
acid CH3-CH2-COOH
colorless liquid with a sweet odor; preservative
- Ethyl
mercaptan CH3-CH2-SH
"rotten
eggs" - the smell of natural gas
Chemical building
blocks* & Biomolecules
summary table*
back next
lecture
 key
concepts* key
concepts*
copyright c2021
Charles Mallery,
Biology 150, Department of Biology,
University of Miami, Coral Gables, FL 33124
-
VView@homee
SKIP ALL THE MATERIAL
BELOW...
Conformation - [3D-shape]... the
surface outline or contours...
3-D orientation of
a molecule, which results without
breaking any covalent bonds |
due to
free rotation [360o]
of atoms about
a single chemical bond
& the weak
electrostatic forces holding
molecules together... figure* |
►
STRUCTURE, SHAPE
and
FORM
and
BIOLOGICAL
ACTIVITY... |
Molecular
shape is CRUCIAL
in biology, because it determines how
biological
molecules recognize
& respond to
one another with Specificity.
Only molecules with
complimentary shapes can form weak bonds with
each other:
ex: opiates &
endorphins -
fig
2.16*
- morphine is an opiate
isolated
from opium and heroin, from which it is
made. In 1975 endorphins
(signal molecules of pituitary that bind to
brain receptors relieving pain)
&
opiates, as morphine, were shown
to have molecular shapes
similar to
each
other, and they can mimic
them by binding
to endorphin
receptors. |
|
|
Molecules may have many
shapes or forms...
but How does shape
influence biological
activity?
|
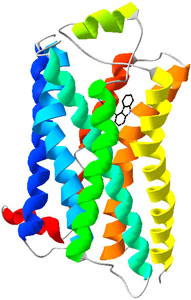
adrenergic receptor |
When one of the vast
numbers of molecules milling around
in our cells (adrenaline) is of precisely
the correct shape and
decorated with the right charges, it binds to a
receptor
where
it can produce a vital reaction as making
a heart beat faster. |
Beta-1 receptors
are found in the myocardium
(heart muscle) & leads to
cardiac stimulation.
Beta-2
receptors are found in smooth
muscle,
skeletal
muscle, and the liver.
They are involved
in bronchodilation and vasodilation.
|
|
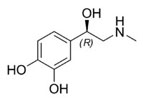 |
adrenaline
(epinephrine) |
 |
The 3-dimensonal shape of a receptor protein
(<-- as the beta-2 adrenergic
receptor) within a cell
allows the specific binding of signal molecules,
here adrenaline (adrenaline/adrenergic
receptors)
|
Leptin*
- the fat hormone
polymers
2. Ibuprofen
& Albuterol occur
as R & S enantiomers, which
characterize
the configuration of the whole molecule, not a
specific stereocenter (NH2).
Only
one of two stereoisomers, R or S, is effective - fig
4.8*
|





