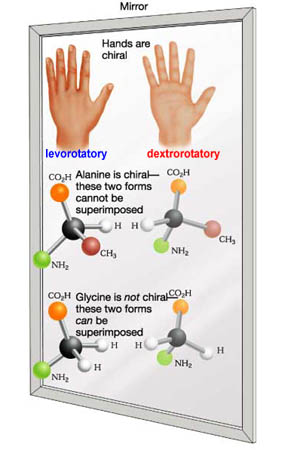
Enantiomers are molecules of identical elemental composition, but are not superimposable upon each other structurally. Enantiomers are then two stereoisomers that are mirror images of each other and exhibit opposite spatial configurations and are known to be chiral molecules having different optical activity.
One unusual property of enantiomers is that one purified form isomer will rotate the plane of polarized light a set number of degrees to the right. This is called the Dextrorotatory isomer or (+) isomer, and sometimes by the configuration as the "R " isomer from latin root dexter, "right-handed", or rectus, "right, correct, upright".
The other enantiomer will rotate the plane polarized light the same number of set degrees in the opposite left direction. This isomer is said to be a Levorotatory isomer or (-) isomer; also known by configuration as the "S" isomer from latin root, sinister, "left"].