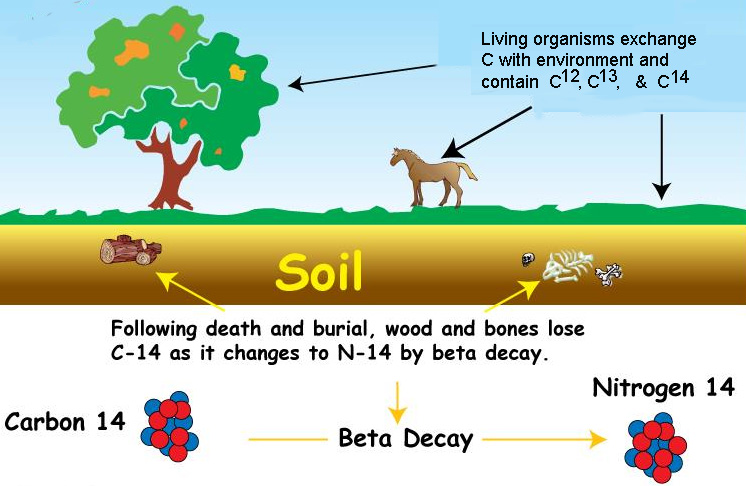
Once an organism dies
(plant, animal or their by-products), it
ceases to absorb 14C, so the amount of radiocarbon present in its tissues at death
steadily decreases from
that point on with the time frame of
14C's
half-life. 14Carbon
has a half-life of 5,730
+/- 40 years i.e.,
half the amount of the radioisotope present at any
given time will undergo spontaneous disintegration
during the succeeding 5,730 years. A half-life
is the time it takes for one-half of the parent
isotope to decay* to its daughter isotope
(14C to 14N). Because 14Carbon decays at the
constant rate of 5,730 years, an
estimate of the date at which an organism died
can be made by measuring the amount of its residual
radiocarbon
remaining.
The 14carbon method was
developed by the American physicist Willard F. Libby about 1946. It has
proved to be a versatile technique of dating fossils and
archaeological specimens from 500 to 50,000 years
old. The method is widely used by Pleistocene
geologists, anthropologists, archaeologists, and
investigators in related fields.
back
Carbon dating equation |