The Chemistry
of Life involves...
the reactivity of the
Molecules in Living Systems
The STRUCTURE
of biological molecules and their SHAPE determines the roles
they play in
the complex chemical processes of LIFE;
yet even the most complicated
biological molecules can be divided into
smaller and smaller
FUNCTIONAL GROUPS*
All the presentation
material below is for review of freshman biological
chemistry
Elementary Chemistry is based
upon...
Periodic
Table
- The Periodic
Elements are matter composed of ATOMS with an
identical number of Protons
which cannot be reduced to simpler
substances by normal chemical means, and
only 30 of the 92 natural
elements commonly occur in Living Systems.
99% of Living Matter is made of
C H O P S N
All have low atomic
numbers ecb fig
2.7*
Table ecb 2.5 (valance
electrons)*
and are very reactive forming covalent*
bonds with precise 3D
geometries*.
| Molecular composition of
cells... |
|
Water (H2O)

|
70 % |
|
Inorganic
ions (as...Na, K, Cl, PO4) |
1 % |
|
Small monomers (amino acids,
sugars, nucleotides) |
5 % |
|
Macromolecules (protein,
nucleic acids, etc) |
24 % |
| chemical composition
of bacteria ecb 2.27*
|
|
 |
|
let's look at small
biomolecules [MONOMERS] made
via Chemical Bonds & Functional Groups
[Review Panels 1 to 7 in chapter 2]
Small biomolecules, the monomers, [ecb 2.28*] that make
the cellular biopolymers.
a. Hexose
sugars
- compounds with
repeat formula of...
[CH2O]n
aldoses vs. ketoses*,
rings*, alpha & beta-links*, isomers: glucose vs.
galactose*
 *
glucose
+
glucose = mono-,
disaccharides*, tri-,
polysaccharides* *
glucose
+
glucose = mono-,
disaccharides*, tri-,
polysaccharides*
&
long chain
polymers of monosaccharides
b.
Fatty
Acids - fatty acids:
saturated
FA vs unsaturated*
[Table
2.4]
form triacylglycerols* =
lipid - 3 chain hydrocarbons*
animal fats* & trans fat
 * and
phospholipids*
easily assemble into membranes*) * and
phospholipids*
easily assemble into membranes*)
easily
self-assembly into aggregates*:
soap micelles &
bilayers*
with fluidity*
fats also include steroid
& cholesterols*
(4-ring skeleton) as lipids because
they're insoluble
& occur
in membranes (mcb fig 1.13)*

1st
amino acid discovered was asparagine (1806 in
asparagus)
last amino acid described
was threonine
(1938) |
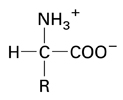
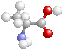
STRUCTURE*
- amino acids have a carboxyl group
(-
COOH)
&
amino group
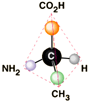
(-NH2) ...bound to an asymmetric carbon
20
ubiquitous aa's have 4 groups on α -C in a tetrahedral shape
|
|
charge of Amino Acids
-
an ACID molecule that tends to
release a H+ (-COO-)
& a BASE with a group that
readily combines with a H+ (NH3+) |
Zwitterion - an
ampholyte* molecule carries
charges on different groups, as an
amino acid,
but is neutral;
[amphoteric - can react as either an
acid or
base] |
|
Isoelectric
Point
- pH where no net charge
in molecule |
|
pK (table of aa
pK's) - pH where groups
are 50% ionized & 50% non-ionized |
|
classes of amino
acids...
[classified by chemistry of R-Groups]
polar
charged |
ACIDIC*... negatively
charged
ASP & GLU
R group with
2nd COOH
that ionizes above pH 7.0
|
BASIC*... positively
charged
LYS,
ARG, HIS
R group with 2nd
amide that protonates
below pH 7.0
|
|
polar
uncharged
|
POLAR
UNCHARGED*...
SER,
THR, ASN, GLN, TYR,
are soluble in water,
i.e., hydrophilic
|
nonpolar

aromatic
|
NON-POLAR*... (aliphatic) ALA,
VAL, ILE, LEU, PHE, TRP
contain only
hydrocarbons R groups =
hydrophobicity |
AROMATIC &
SPECIAL*
TRP, PHE,
TYR, GLY, PRO, CYS
contain R groups with
ring structures
& others
|
|
Table of polar
vs. nonpolar amino acids*
|
 next
- Molecular Shape
practice next
- Molecular Shape
practice  questions (ans)
questions (ans)
SKIP
ALL THE MATERIAL BELOW THIS POINT...
There
are only 3 known ways to make a peptide
bond...
1.
chemical abiotic
synthesis in the laboratory...
PPTI
2.
genetic engineering cloning
mechanisms...
humulin
3. biologically, in
cells... (@ rate of
25aa/sec in
prokaryotic cells)
Some common
peptide terminology:
dipeptide,
tripeptide,
pentapeptide,
oligopeptide, polypeptide...
protein - polymer of a-L-amino acids joined by peptide
bonds
ways of depicting
polypeptides: Ras*
whale
myoglobin - ecb2.e panel 4.2 pg
132-133
 next
lecture* next
lecture*
SKIP THIS
TABLE and all material below this
point
examples of naturally
occurring small
oligopeptides - [many are
vertebrate hormones] |
| Nutra Sweet - a
dipeptide*
(2aa)
of L-aspartyl-phenylalanyl-methyl
ester...
aspartame |
insulin
- two
polypeptides...
controls carbohydrate metabolism
1.
alpha
chain
of 30 aa’s
&
2. beta chain of 21
aa |
| glucagon
- pancreatic hormone 29
aa... opposes insulin
action |
| corticotropin
- 39aa -
anterior pituitary hormone...
stimulates adrenal cortex |
| oxytocin
-
9aa
-
hormone of posterior
pituitary... stimulates
uterine contractions
|
| bradykinin
- 9aa
– hormone acts on smooth
muscle... vasodilatation/inflammation |
angiotensin -
octapeptide
(derived from angiotensinogen by kidney enzyme
renin)
-
increases blood pressure
ACE
Inhibitors
block AT & lower bp. [sport] |
thyrotropin
relasing factor (TSH)
- 3
aa’s [Glu-His-Pro] of hypothalmus...
- stimulates thyroid to
release thyroid hormone |
enkephalins -
either of two penta-peptides with opiate
& analgesic
activity.
endorphins -
pituitary made opioid
polypeptides; produce analgesia.
 next lecture*
e5-s15
next lecture*
e5-s15 |
|
|