The Design of Metabolism:
key concepts*
How
Cells Transform Energy...
or How Biological Order Comes About,
METABOLISM...
(Gk -metabole - meaning change)
is mostly the catalytic reactions (run by enzymes)
in that occur in cells,
& commonly
via metabolic pathways
[A --> B --> C --> D -->
E]
Two broad descriptions of cellular
metabolic reactions*
are Exergonic/Endergonic
also,
metabolic reactions are often described as...
-
ANABOLIC... Biosynthetic reactions:
often visualized in... photosynthesis*
- energetically un-favored
reactions coupled with favored reactions
1)
PHOTOSYNTHESIS - reduction of CO2 to CH2O
& synthesis of ATP & NADPH
-
CATABOLIC... Chemical oxidation
of food stuffs via Cell Respiration*
1) DIGERSTION of
POLYMERS (carbs
often to glucose)
via hydrolysis
reactions,
2) GLYCO-LYSIS converts glucose
---> pyruvate [anaerobic splitting of glucose]
3) KREBS
CYCLE
aerobic
oxidation of Acetyl-CoA
---> CO2 + H2O ---> NADH
-
4) ELECTRON
TRANSFER
--->
NADH + O2 ----> H2O + H+gradient
5) ATP
SYNTHASE
---> uses H+
gradient to phosphorylate P + ADP ---> ATP

-
-
-
ENERGY
TRANSFORMATION...
is a key to understanding cellular
metabolism.
It is the change from one type of energy
to another type while making
something happen, which often also
releases useless energy as heat. |
Energy concept:*
often defined as capacity or ability to
do work and
work is a measurement of
change in a system over time.
Kinds*:
POTENTIAL - stored energy;
capacity to do work (eventually);
KINETIC
- energy of motion
CHEMICAL
- electron bond energy
ELECTROMAGNETIC
- photon light energy from the Sun
HEAT - assoc with movement
of molecules in a body of matter;
most random form of energy (wasted).
|
 |
Examples:
general: heat,
light, sound, mechanical.
biological:
synthetic, osmotic, motion.
|
|
-
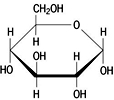
Molecules in living cells
have chemical potential
energy
to do work,
- because
of the arrangement (orientation) of
their atoms in space...
-
we call this CHEMICAL BOND ENERGY because
- the
energy in cells is stored in the
COVALENT BONDS of their molecules.
|
 |
most
cellular energy is needed to maintain...
HOMEOSTASIS*
 a steady state condition that transforms
energy
a steady state condition that transforms
energy
and
keeps cell's away from equilibrium. |
|
-
-
BIOENERGETICS -
is study of energy transformations
(changes) in
Biological Systems & is based
upon...
EQUILIBRIUM
THERMODYNAMICS... is the systematic study of
transformations of matter
and energy in systems in terms of a concept called
thermodynamic equilibrium,
where the word equilibrium implies a state of
balance (thermal,
mechanical,
and chemical equilibrium) that does not change with
time and space.
-
1st LAW = Conservation of
Energy... energy is a constant:
energy can not be created nor destroyed, only transformed.
experimental CALORIC DATA
says this LAW is a true hypothesis.
► Calorimetry* of glucose releases
heat = -686 Kilocalories/mole
(180 amu).
In cells oxidation of glucose yields
free energy: ΔG°′
= - 686 Kcal/mol.
To capture this free energy
in a usable form cells employ a
series of metabolic steps coupled to the
synthesis of ATP.
2nd LAW = Energy transformations favor reducing the
order of the universe:
thus entropy = is a measure of disorder in a system -
ENTROPY*
Entropy
is
directional ---> toward equilibrium (toward
maximum disorder)
and may
define time - a change of one observed
status to another status
The Rules of Universe and equilibrium
thermodynamics are simple :
the natural tendency is for disorder to increase
spontaneously.
Cities crumble, stars go Supernova, & we're
all equilibrizing
(dying...)
Law of ENTROPY says... Degree
of disorder of the Universe
-
(its randomness - its entropy) CAN ONLY INCREASE.
-

Do
Cells obey Laws of Chemistry, Physics, &
Thermodynamics?
CELLS appear not to... WOW ! ...they
become more highly
ORDERED [grow & divide].
Fertilized
egg
---> dividing
cells,
wing
of bird, a spider's web, the human
eye,
all from a
fertilized egg cell... which Grows & Differentiates. we get more order: HOW?
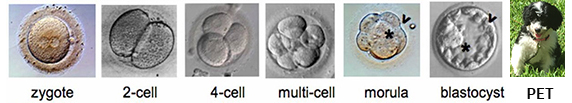
for one part of a system to
become more ordered - lose entropy - (such as a cell),
its
surroundings must become more disordered -
gain entropy...
Cells exchange Materials & Energy with
their surroundings...
|
FOODs
(light & covalent bond energy)
|
 |
cell
reactions give increased order via energy
transformations
|
 |
|
but with release of
HEAT
|
 HEAT (most disordered form of energy)
= max entropy
HEAT (most disordered form of energy)
= max entropy
ENERGY IN
----> CELL
STRUCTURE
----> ENERGY OUT
How do we measure the energy changes
of metabolism and reactions
in cells...
Josiah
Gibbs (Yale 1880's) devised applications of
thermodynamics to chemical reactions...
FREE ENERGY
Equation ΔG
= ΔH
-
T
ΔS
free
energy enthalpy
entropy
-
ΔG is measure of change (Δ) in amount energy in
a system that is ABLE
TO SOME WORK...
ΔG is a numerical measure of how far a chemical reaction is
from equilibrium...
-
► Entropy Increases (Disorder Increases)... when useful energy,
that which could be used to do work, is dissipated
as heat...
-
ΔH - enthalpy is
internal heat, often measured as heat
released in a reaction.
-
but, cells are ISOTHERMAL: (-2o to 80oC many @ 37oC) -
thus ΔH
above
≈ 0
Enthalpy may be thought of as heat
content of a system.
-
Cells function within a very narrow temp range
[23o-37oC],
and thus, biologically ΔH is negligible in
the equation;
so, ΔG in cells then is = T
x ΔS the entropy of
the system; its degree of disorder.
-
thus, ΔG can PREDICT... the
Direction of Cellular Reactions... TOWARD EQUILIBRIUM...
-
Toward
Maximum ENTROPY
& Toward a Release of
Free Energy.

-
-
-
-
-
-
-
-
-
-
-
-
-
in a BIOCHEMICAL REACTON
A
<---> B
Which Way is toward more Disorder?
We need to be able to measure the change in Entropy [ΔS] of a
reaction... but
how?
A
derivation of Gibbs Free Energy [ ΔG
= ΔH - T ΔS
]
equation can help here:
ΔG = ΔG0’ + RT ln [p]/[r]
change in free energy content
of a reaction ( ΔG )
...depends upon:
1. energy stored in molecule's covalent bonds
2. temperature is negligible... i.e., as cells
are isothermal,
thus...
ΔG
= actual free
energy at any
time during course of a reaction
ΔGo'
= standard
free energy... free
energy change under specific conditions]
when 1 mole of substance is formed at 250C,
1 atm, etc...
a measure of how far a reaction is from
equilibrium.
R
= gas
constant (2 x 10-3
Kc/mol)
T
= absolute
temp (2730K + 0C)
ln
= natural log (conversion to
log10
= 2.303)
3. at
equilibrium by definition ΔG = 0 & we can call
ratio of [p]/[r]
=
Keq
equilibrium
constant
 thus...
thus...
Let's solve the Free Energy
Equation for Standard Free Energy
ΔG0' ...
ΔG
= ΔG0'
+ RT ln [P]/[R]
@ equilibrium ΔG
= 0
thus 0
= ΔG0' + RT ln [P]/[R]
& rearranging
ΔG0' =
- RT ln [P]/[R]
@ equilibrium [P] / [R] is referred to as the Keq, the Equilibrium Constant
& @ 250C ... -RT ln
Keq
= - (2.0)
(298) (2.303) lg10 Keq =
-[1372] lg10Keq
... thus ΔG0'
= - [1372]
lg10Keq
R = gas
constant ( 2 x 10-3 Kc/mol)
T = absolute temp
(2730K + 0C)
ln = natural log (conversion to
log10
=
2.303) .
So now we can measure ΔS [entropy] by
observing the ratio of the
CONCENTRATIONS of
reactants & products...
by solving the above equation for
ΔGo' we can see the
relationship*
of Keq
to ΔGo'

The difference between
ΔG (actual free energy)
and ΔG0' (standard free energy)
ΔG0 is a fixed value under
idealized conditions for a given reaction
conditions,
[1 mole reactants
/ 250C / 1 atmosphere] and indicates in which
direction
that reaction is likely to proceed toward
equilibrium. o
Cells often
employ hydrolytic reactions to add energy to
unfavorable reactions.
phosphoryl hydrolysis*
and table
of Phosphoryl hydrolysis*
Standard conditions do
not exist within a cell, but ΔG0' is
useful to
predict the likely
direction of
a specific reaction (exergonic
or endergonic).
ΔG
is determined by the concentrations
present at any given time during
a reaction
and is a measure of how far a reaction is
from equilibrium at that time.
Cell metabolism (Life) is
essentially a non-equilibrium condition.
► Metabolism works by changing the relative
concentrations of reactants and
products that influences
the progress of non-favored catalytic
reactions.

CHEMICAL
REACTION A <----> B
Which Way & Why?
EXERGONIC REACTION - is one which releases free energy [
-ΔG ]
Product (B) <<< energy REACTANT (A) [energy stored in covalent bonds
is lost]
ex: burning
wood (cellulose)
glucose polymer = chemical potential energy.
breaks bonds, release heat & light ---> CO2
& H2O
fig
9.4*
cell respiration -
cellular burning of glucose molecules.
slower, multi-step process that captures
& conserves some energy... as ATP
ENDERGONIC REACTION - requires input of energy for
A --> B
Product (B)
>>> energy than REACTANT
(A)
[ +ΔG ]
ex: photosynthesis
(autotrophy)
glucose
is made from CO2 +
H2O
--light--> C6H12O6
energy poor vs.
energy rich
Exergonic vs.
Endergonic summary
----> LIFE and EQUILIBRIUM*...

CELLULAR METABOLIC REACTIONS are
then a mix of...
Exergonic
& Endergonic
reactions that occur inside of cells...
How does Cell Metabolism
really work energetically within cells?
for RX's which share one or more
intermediates (a pathway)...
[ A --> B --> C
--> D = -ΔG ]
the overall
free energy change (ΔG) is the sum of indiv ΔG's
ΔGo'
Glu + Fruc-6P
---> Sucrose
+ 5.5 Kc/m =
not favored
ATP*
---> ADP + P
- 7.3 Kc/m
= favored
Glu + UTP
---> UDP-G + Fruc-6P
--> Suc-6P + UDP - 1.8Kc/m
(the reaction*)
COUPLED
REACTIONS*
- often involve... the linking of the hydrolysis
of ATP
(a favored
rx) to a
thermodynamically unfavored reaction,
thereby creating
biological order
(greater molecular structure).
 another
ex: synthesis of glutamine*
another
ex: synthesis of glutamine*
-
Why is ATP the
"molecular currency" of cell energy
transfers?
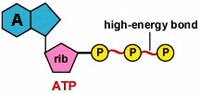
... the ENERGY MOLECULE of CELLS
is ATP
over the period of evolution,
cells favored
enzymes
that
bound ATP & used its
hydrolysis to drive endergonic rxs?
... but, couldn't any
nucleotide work?
Was there a
1st ATP?*
|
 * * |
-
adenosine
triphosphate*
- its structure* is
its source of energy...
1. electrostatic
repulsion 2.
resonance 3. sphere
of hydration...
copyright c2024 Last update
- March 2024
Charles Mallery,
Biology 150, Department of Biology, U.
of Miami, Coral Gables, FL 33124
|






