How
Cells Make ATP... denovo
pathways for bases and ribose sugars or salvage
reactions.
Salvage: by
PHOSPHORYLATION... adds
a phosphate to ADP
ADP + P
<------>
ATP
(recycle*)
cell ratio of ATP/ADP is about 10:1 (a
non-equilibrium state) |
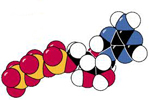 |
How do cell's phosphorylate ADP
to make ATP?
a) Substrate
Level Phosphorylation...
where a substrate molecule ( X-P ) donates* its P to ADP making ATP fig
9.6*
b) Oxidative
Phosphorylation... involves capturing e-'s
for use in Electron
Transfer Chain...
1.
food molecules donate e- & protons to acceptor molecules via oxidation NADH*
2.
NADH passes on electrons &
protons are pumped out of
mitochondria...
(or the chloroplast
membranes in
photosynthesis);
3. protons diffuse back into
mitochondria thru an enzyme = ATP
synthase,
the
ATP synthase
enzyme
makes
ADP + P --> ATP
figure*
c) Photophosphorylation...
e- of light energy, instead of food covalent
bonds, are captured by chlorophylls
to make a proton
gradient across the
chloroplast
membranes...
figure*
 protons move through a
chloroplast ATP synthase enzyme to make ATP
protons move through a
chloroplast ATP synthase enzyme to make ATP |
1st
- Oxidative
Metabolism...
(Cell Respiration in animal
cells)
occurs in
heterotrophic organisms that consume foods
... we say organisms oxidize (consume) foods
(often glucose) to make energy
because it
removes
& captures electrons...
... where is energy in
foods? it's in the covalent bonds (e-)
thus = oxidative
metabolism is... cells capturing
e-
via REDOX reactions.
what is a
REDOX REACTION*... methane
example - fig 9.2*
in
step
6 of glycolysis
e-
passed from PGAL --> to
diPGA*
energy is transferred via a redox couple with NAD+
by e-
OXIDATION =
removal of electron &/or protons from
food covalent bonds
REDUCTION
=
gaining electron &/or protons; adds
an electron to an acceptor molecule
Redox state*
of molecules
--> the more
reduced = the more energy it holds

a model
redox reaction...
A-H +
B-O
<--->
A +
B-O-H
donor
acceptor
(:H)
acceptor
donor
Let's look at a
redox reaction in the Krebs
cycle pathway using NAD+.
isocitrate + NAD+
< ---
> alpha-ketoglutarate* +
NADH
reducing
oxidizing
becomes
becomes
agent
agent
oxidized
reduced
a 2nd redox coenzyme (couple)
of living cells is
FAD+
<--> FADH2*
acceptor
reduced
Transition metals [metallic
elements with unfilled valances that accept e's...
Cu, Fe,
Zn, Mn] and may have been involved in origin
biological redox reactions
and
evolved into today's oxidoreductase (dehydrogenase)
enzymes?
Structural similarity suggests
an early origins of biological electron transfer*
Thus : heterotrophic
metabolism is the stepwise OXIDATION
of molecules (foods)...
if AEROBIC - directly requires oxygen directly as the electron acceptor
if ANAEROBIC - requires no oxygen directly
(uses other e- acceptors)

CELL RESPIRATION... is the
oxidation of food stuffs that produces ATP.
Overview
of Cellular Respiration*
1. oxidation
of
GLUCOSE (6C) -->
PYRUVATE
2 (3C) --> GLYCOLYSIS
2.
oxidation of pyruvate --> CO2 + H2O
--> KREBS CYCLE
3. reduction O2 to H2O
--> ELECTRON TRANSPORT CHAIN
C6H12O6
+ 6O2
<----> 6 CO2 + 6 H20
+ e- --->
36-38 ATP
ΔG = -686 Kc/mole
-270Kc = 39%
called oxidation... because e- are removed from glucose
called reduction... because e- passed to O2 making water
4. phosphorylation of ADP via
ATP synthase
thus the terminology... oxidative
phosphorylation [chemiosmosis]
 Cell Respiration is
a multi-pathway process*...
Cell Respiration is
a multi-pathway process*...
a more complete description
of Cell Respiration :
-
series of enzyme rx's (in biochemical pathways) in the cytoplasm & mitochondria
(glycolysis, fermentations, pyruvate oxidation,
Krebs cycle, electron transport),
- removes e- (oxidation) from covalent bonds of
substrates (as glucose),
and
- pass e- to acceptor molecules [redox coenzymes]
such as NAD+ &
FAD*
which become reduced to NADH &
FADH2
- the reduces these coenzymes [
NADH & FADH2 ] passing
e- to other e- acceptors...
a series of metalloprotein
Electron Transport carriers, including
cytochromes,
- the electron carriers (cytochromes) pass e- to O2 ---
reducing it to ---> H2O
- while pumping
protons [H+] out of mitochondria into
the peri-mitochondrial
space,
creating a proton gradient
as an diffusive energy source
- the protons move back into mito thru a special
enzyme (ATP
synthase) &
make ATP

let's look at the details of each of
these processes...
all
the details: PATHWAYS of CELL RESPIRATION
which make ATP...
what should we
know - what goes in, what comes out,
and where,
also the types of chemical reactions that
occur in the pathways.
|
|
Glyco-lysis
: 10 step pathway converts 1 glucose (C6) to 2 pyruvate (C3)
products : 2 molecules of
pyruvate, 2 NADH,
& 2 ATP (net)
occurs in : cytoplasm [and is
anaerobic - does not require
O2]
may lead to:
alcoholic fermentation
= glucose --> alcohol
lactic acid
fermentation = glucose
--> lactic acid
|

|
KREBS Cycle :
oxidizes 2
pyruvates from
glycolysis to CO2 + H2O
produces :
8 NADH, 2 GTP (ATP equivalent), 2
FADH2
releases :
6 CO2 (thus
called... Cell Respiration)
occurs in the mitochondria [and is
aerobic = directly requires O2]
|
ETC - Electron Transport
Chain
:
uses e-
carrier proteins (including
cytochromes,
etc...)
to pass e- & H+ from NADH & FADH2 to O2 making H2O
&
generates a proton
gradient (chemiosmosis) across the inner mitochondria
membrane
|
ATP synthase
: a
carrier enzyme in the inner
mitochondrial membrane that
passively transports H+ back into mitoplasm
& while making ATP
directly
 |
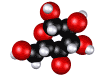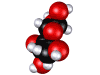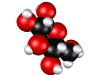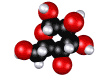
1. GLYCOLYSIS...
KEY
ENERGY REACTIONS of GLYCOLYSIS...
Animation of Glycolysis*
don't memorize the pathway, but learn
the major reaction types...
1.
substrate
level phosphorylation*
[occurs twice*
in glycolysis]
2. redox
reaction step 6* involving
NAD+
3. reaction paths -->
investment phase*
& payoff phase* - Summary of glycolysis*
FATES of
the products:
1. PYRUVATE*...
if anaerobic
- involves alcoholic fermentation
& lactic acid respiration
alcoholic
fermentation*
"history of wines" animation
lactic
acid fermentation*
also called anaerobic
respiration
if aerobic -
involves pyruvate dehydrogenase +
Krebs Cycle + ETC
2. cytosolic
NADH... - it holds captured
e-
energy,
but how some cells can use it?
mito membrane is impermeable to NADH
-> cytoplasmic & mitoplasmic pools
Shuttles* malate shuttle
(liver, kidney, heart) = NADHc --> NADHm
glycerol-P shuttle (muscle/brain)
=
NADHc --> FADH2m
Purpose:
to move electrons captured in cytosolic NADHc into mitochondria |

|
 |
SUMMARY
of GLYCOLYSIS
- 2 ATP
to initiate
-
2
substrate level
phosphorylation steps = 4
ATP gross (2 ATP net) and
-
1 redox
step making NADH
thus
Glycolysis makes:
(figure*)
2
ATP (net),
2
NADH, and
2
PYRUVATES
remember
the role of the ... Fermentations & Shuttles
END OF
MATERIAL FOR EXAM #3 (electron microscopy to here)
 the Glucose songlisten
at home
the Glucose songlisten
at home
Heterotrophic Metabolism (cell respiration) in Aerobic Organisms...
if aerobic then the
Fate of Pyruvate*
is oxidation in mitochondria
1. PYRUVATE
DEHYDROGENASE Reaction... [before Krebs
cycle itself]
in
mitoplasm
(Fig 9.10)* oxidizes
PYR
--> acetyl-CoA
a multienzyme complex of 60 proteins and 5
coenzymes
involves CoASH* --ADP*-->
acetyl
coenzyme-A [Fritz
Lippman]
reactions: 1.
decarboxylation
(-CO2),
2. reduction of 2 NAD+
-->
2 NADH
3.
acylation with
synthesis of 2
Acetyl-coA .
|

|
|
|
2. KREBS CYCLE [Sir Hans Krebs] animation
of cycle* &
cycle
summary*
Key Reactions
full
cycle details
& enzymes
involved
1.
NAD+ is reduced (6
NADH)
and FAD is also reduced (2 FADH2)
2.
substrate level phosphorylation
occurs (2
GTP <--> 2 ATP)
3.
decarboxylation occurs -4x [-COOH]
4.* 2 acylation
reactions via coenzyme-A (forms
2 Succinyl-coA)
|
 -->
how
many actual
ATPs per glucose are
made so far?
only 4 (net) by SLP -->
how
many actual
ATPs per glucose are
made so far?
only 4 (net) by SLP |
ELECTRON
TRANSFER CHAIN & OXIDATIVE PHOSPHORYLATION
the coupling of oxidation of substrates (-e) to phosphorylation of ADP to make ATP
.
► remember, most of the energy
of glucose's bonds is now carried in NADH & FADH2
e- are passed from NADH/FADH2
to O2 (aerobic) ► ETC animation*
these series of electron
carrier proteins
occur in 4
membrane subunits
fig 9.14*
I)
NADH
Reductase,
II ) Succinate Dehydrogenase
III)
Cytochrome
Reductase,
IV) Cytochrome Oxidase
CHEMIOSMOSIS: hypothesis
of e- transport createing a PROTON
MOTIVE FORCE - figure*
e- carriers release protons
into perimitochondrial space
making a proton gradient.
1978 Nobel Prize (Chemistry) to Peter Mitchell for
Chemiosmotic Hypothesis.
Can a hydrogen ion gradient
(H+) created by e- flow thru the ETC
also make ATP ???
- experimental evidence of a
H+ gradient making ATP came via
bacteriorhodopsin*
- H+ diffuse back into mitoplasm thru
ATP synthase ---> ATP
via a molecular motor*
-
Boyer
hypothesis* = chemical to mechanical
chemical energy (like a water wheel)*
- animation
of ATP synthase action*

Overview of
Cell Respiration*...
► A rule of thumb for the
amount of ATP made
per e- pair...
the Phosphate/Oxygen Ratio, or (P/O ratio),
refers to the amount of ATP produced
from the movement of 2 e's
through the ETC to the reduction of an oxygen
atom.
Measured empirically it
is --> NADH ≈
3 ATP
&
FADH2 ≈
2 ATP
So How much ATP can be made per Glucose...
the ATP/O2 ratio... based on 3 ATP per NADH & 2 ATP per FADH2
idealized = 36 to 38
ATP [ a yield table ]
What can serve as substrates for aerobic
metabolism?*
carbohydrates, proteins, lipids... can serve as substrates
Aerobic metabolism
intermediates are precursors for other molecules*
How is
heterotrophic metabolism regulated?
1. stiochiometric [substrate
concentration] levels
is a main control mechanism
2. allosteric controls include: +AMP/ADP stimulate enzymes
& +ATP
inhibits enzymes
Key mammalian allosteric enzyme :
PHOSPHOFRUCTOKINASE*
feedback
inhibition
allosteric regulation

SUMMARY
- Heterotrophic Metabolism - CELL RESPIRATION
1.
Substrates = sugars,
amino acids acids, fatty acids
2.
Pathways
= Glycolysis, Krebs Cycle, ETC, & ATP synthase are Universal to cells
3.
Products = C02, H20, and energy as H+ gradient,
NADH, FADH2, &
ATP
4. part of
process is Anaerobic (doesn't require -02 ; Glycolysis)
&
may include alcohol & lactate fermentations (...anaerobic
respiration)
& part is Aerobic (requires +02 ...electron transfer
chain),
► 5.
Reaction types include:
oxidations, reductions, substrate
level phosphorylations,
decarboxylations, acylation, oxidative phosphorylation,
& hydrolysis (or dephosphorylation)

6. Energy capture is
via electron transfers, proton pumps,
H+
gradients,
& ATP
synthase*
7. Regulation is by:
stiochiometry, feedback inhibition
& allosteric modulation
(key
enzyme: phosphofructokinase)
8. Intracellular compartmentation:
glycolysis occurs in the cytosol
Krebs Cycle is mostly in the mitoplasm of mitochondria
ETC is in the cristae
membranes of mitochondria
ATP synthase
within the inner mitochondrial cristae membrane
9. Cell
Respiration is
KEY and
central to all of a cell's metabolic pathways
IUBMB-Nicholson Metabolic Pathways Chart
back
next
lecture* -
photosynthesis
 Key Concepts* Key Concepts*
copyright
c2023
Last update - March 2023
Charles Mallery,
Biology 150, Department of Biology, U.
of Miami, Coral Gables, FL 33124
SKIP THE MATERIAL
& LINKS BELOW...
If
you have comments or suggestions, email me at
cmallery@miami.edu
oxidative
phosphorylation animation
-
Individual reaction steps of glycolysis
anim
ATP synthase rotary mech - side
& top
- ATP
synthase & H-Gradient make ATP*view@home & BioVisions-Powering
the cell*view@home
animation of ATP synthase
action
know what goes in & come out*
Cancer reprograms energy metabolism
"what
are these carrier molecules*"
(figure)
|



