NAD+ + 2H+ + 2e- <---> NADH + H+
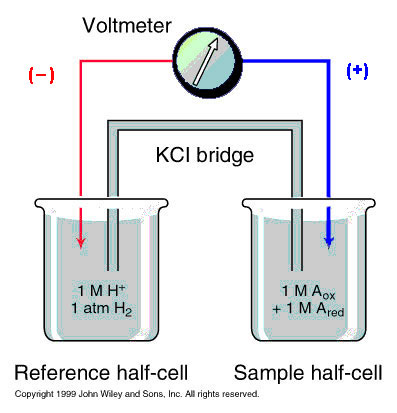
The other beaker contains the hydrogen reference standard ( H2 <-->2H+ + 2e-), whose redox potential is arbitrarily set to ZERO by international agreement. A salt bridge is formed from a concentrated KCl solution allowing the ions K+ & Cl- to move between the two beakers to neutralize the charges between them. The metal wire (red) provides a resistance-free path for electrons, and a voltmeter measures the redox potential of substance A.
If electrons move from Ared to H+, as shown, the redox couple has a negative redox potential. If they flow from H2 to Aox, this couple has a positive redox potential.
thus, theremodynamically e-'s flow from...
more electronegative to more electropositive