Polynucleotide
Phosphorylase:
In 1955,
Marianne Grunberg-Manago
and
Severo Ochoa
reported the isolation of an enzyme that catalyzed the
synthesis of RNA. Their work built upon the earlier work of
Jerard
Hurwitz & J.J.
Furth who
performed experiments to see if isolated
E. coli protein fractions could polymerize radioactively
labeled nucleotides. Later
Grunberg-Manago
& Ochoa
tested a protein fraction that could make RNA. For this work,
Ochoa
shared the 1959 Nobel Prize
(but not Grunberg-Manago) in Medicine with Arthur
Kornberg
(who received the Prize
for his work on DNA polymerase I).
Their enzyme could convert ribonucleoside
diphosphates into
RNA:
(RNA)n
+ NDP --►
(RNA)n+1
+ Pi
However, the enzyme had a number of
unsettling properties. It did not need a template; and, it
could use as little as 1 NDP or as many as 4 NDPs as
substrate. In fact, the sequence of the product RNA depended
entirely on the number and concentration of substrate
NDPs. These are not the properties of an enzyme that
must faithfully copy the genetic material for
expression! We now know that Grunberg-Manago
and Ochoa
had isolated the enzyme
polynucleotide
phosphorylase
which usually
catalyzes the breakdown of RNA - not its synthesis! i.e.,
its a ribonuclease.
(RNA)n
+ NDP ◄--
(RNA)n+1
+ Pi
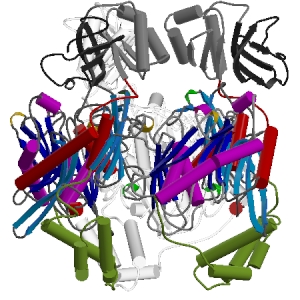
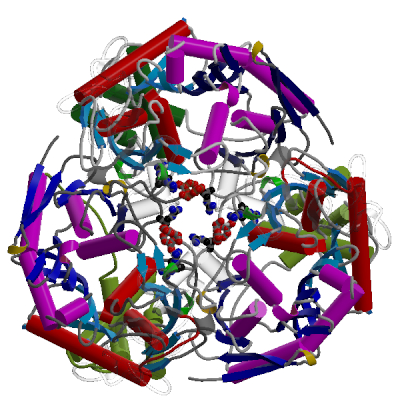
Polynucleotide Phosphorylase
Authors: M. F. Symmons, G. H. Jones &
B. F. Luisi
Reference: Structure, 8:1215-1226,
2000
Description:
PNPase is a disk-like trimeric exoribonuclease.
This side view of the trimer shows the interface
between two subunits. The cores of subunits are constructed
from two homologous domains shown in shades of red and blue.
The lower and upper accessory domains are in green and grey
respectively.